Chemistry often uses compact formulas to represent complex reactions. The phrase hcooch ch2 h2o looks unusual at first, but it reflects a combination of fragments that point to ester hydrolysis. This reaction involves the breakdown of an ester in the presence of water, leading to the formation of useful products such as formic acid and alcohols. To make sense of hcooch ch2 h2o, we need to explore what it represents, how the reaction works, why it is significant, and where it is applied.
What does hcooch ch2 h2o mean?
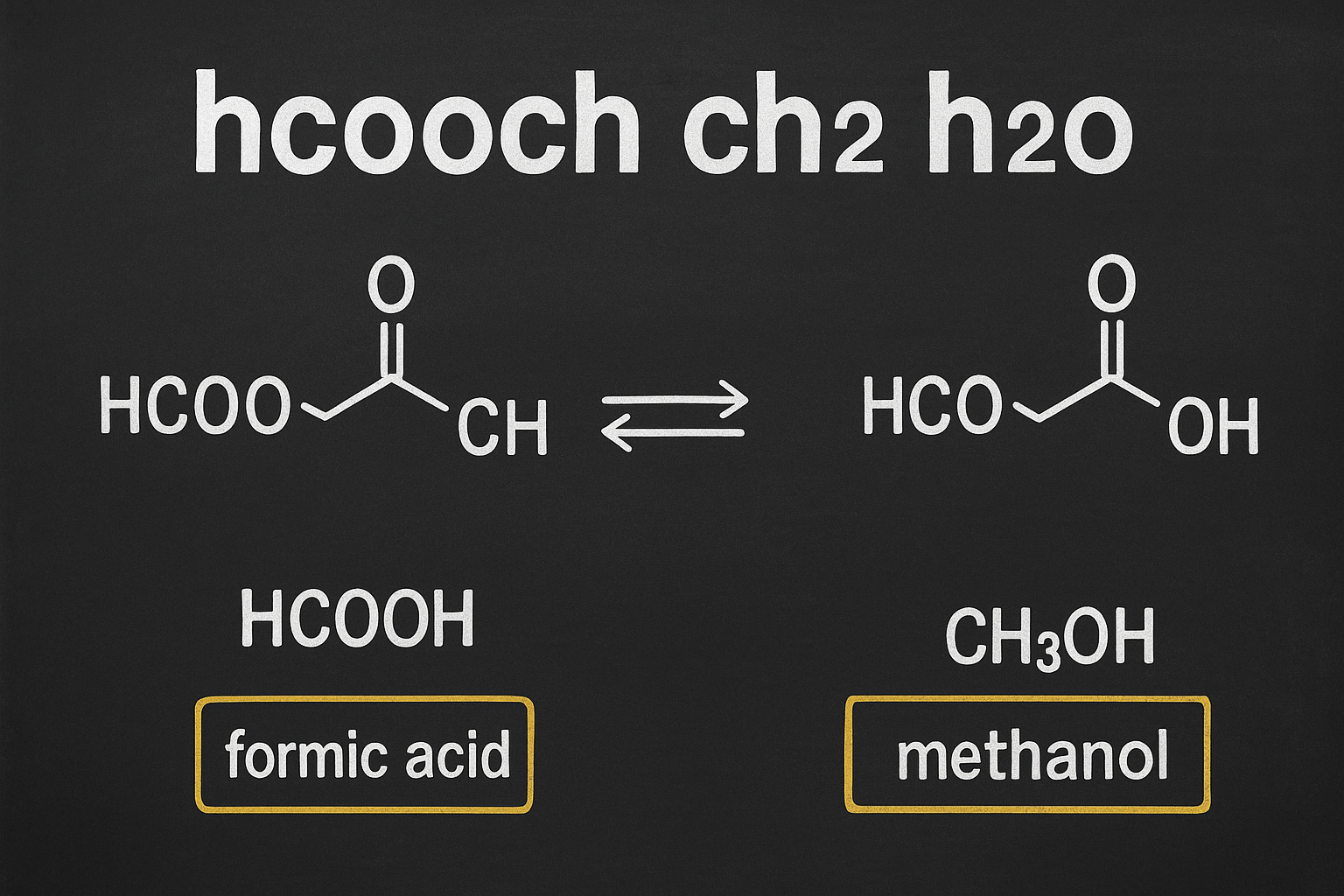
When chemists read hcooch ch2 h2o, they often recognize it as shorthand for a formate ester reacting with water. In practice, this is most commonly interpreted as methyl formate combined with water, which undergoes hydrolysis. The products of this process are formic acid and methanol. This simple transformation highlights the principle of ester hydrolysis, which is central to both laboratory chemistry and industrial production.
The Chemistry Behind hcooch ch2 h2o
The balanced reaction that hcooch ch2 h2o points to can be written as:
HCOOCH3 + H2O ⇌ HCOOH + CH3OH
Here, methyl formate reacts with water to form formic acid and methanol. This is a reversible reaction, meaning it can proceed in both directions depending on the reaction conditions. In practice, catalysts and environmental factors like temperature and pressure play an important role in controlling which side of the equation dominates.
Reaction Mechanism
The mechanism of the reaction represented by hcooch ch2 h2o follows the typical pathway of ester hydrolysis:
- Under acidic conditions, the carbonyl group of the ester becomes protonated.
- Water attacks the carbonyl carbon, forming a temporary intermediate.
- The intermediate breaks down, producing formic acid and methanol.
Under basic conditions, hydroxide ions attack the carbonyl directly, creating a similar intermediate before releasing the final products. Both pathways highlight how water is essential in breaking down the ester bond.
Industrial Importance of hcooch ch2 h2o
Hydrolysis reactions like hcooch ch2 h2o have strong industrial significance. Methyl formate is used as a starting material in the production of formic acid, which itself is valuable in tanning, agriculture, and preservation processes. Methanol, another product of the reaction, is a widely used industrial alcohol with roles in fuels, solvents, and feedstocks. Managing such a reaction on a large scale requires precise control of equilibrium, separation of products, and safe handling of volatile compounds.
Applications in Research and Industry
The chemical system symbolized by hcooch ch2 h2o is applied in:
- Organic synthesis, where esters are commonly transformed into acids and alcohols.
- Industrial manufacturing, where methyl formate hydrolysis provides a scalable method to produce formic acid.
- Educational laboratories, where the reaction demonstrates core concepts of ester chemistry and equilibrium.
Safety Considerations
Any discussion of hcooch ch2 h2o must include safety. Methyl formate is flammable, methanol is toxic, and formic acid is corrosive. Laboratories and industrial facilities working with these chemicals must ensure:
- Proper ventilation and fume hoods.
- Protective equipment including gloves and goggles.
- Safe storage away from ignition sources.
- Careful waste management and spill response procedures.
Analytical Techniques for Studying hcooch ch2 h2o
Monitoring and understanding the reaction involves various analytical methods:
- Infrared spectroscopy (IR) shows the carbonyl and hydroxyl stretches.
- NMR spectroscopy identifies the hydrogen and carbon environments of the products.
- Gas chromatography (GC) separates and quantifies volatile esters, acids, and alcohols.
- Mass spectrometry (MS) confirms molecular fragments and product identity.
Why hcooch ch2 h2o Matters for Learners and Professionals
For students, hcooch ch2 h2o is a neat example of how condensed formulas capture essential reaction details. For researchers, it illustrates the balance of kinetics, equilibrium, and catalysis in real systems. For industry, it’s a vital step in producing important chemicals safely and economically. The shorthand may look abstract, but its applications are broad and practical.
Conclusion
The formula hcooch ch2 h2o represents more than just a string of letters; it reflects an important chemical transformation that bridges laboratory study and industrial application. Understanding its meaning helps in appreciating the role of ester hydrolysis, the production of valuable products, and the need for safety and precision in chemical processes. Whether you are a student learning basic chemistry or a professional exploring industrial production, hcooch ch2 h2o offers a window into useful, real-world chemistry.
Frequently Asked Questions
Q1: What does hcooch ch2 h2o represent?
It represents the hydrolysis of a formate ester, commonly methyl formate, with water to produce formic acid and methanol.
Q2: Is the reaction of hcooch ch2 h2o reversible?
Yes, it is reversible, and conditions such as catalysts and temperature determine the reaction’s direction.
Q3: What products result from hcooch ch2 h2o?
The key products are formic acid and methanol, both important industrial chemicals.
Q4: Why is hcooch ch2 h2o significant in industry?
It’s important because it provides a method to produce formic acid and methanol, which have wide applications in agriculture, solvents, and fuels.
Q5: What safety issues are linked to hcooch ch2 h2o?
The chemicals involved are flammable, corrosive, and toxic, requiring strict safety measures during handling and storage.